Why India Wants To Halt Use Of AstraZeneca Vaccine
Last updated on March 25th, 2021 at 09:28 am
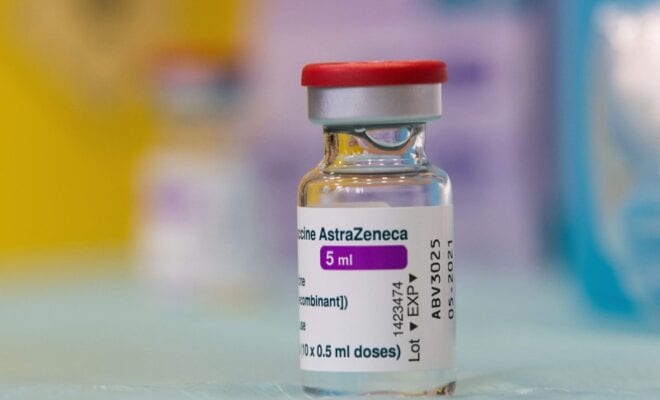
India is now planning to check the after effects of the Astra Zeneca vaccine that is suspected to have been giving patients blood clots. At the moment, India has the choice of almost six different kinds of vaccine variants to choose from.
Some other nations have also halted the intake of the Oxford University developed vaccine variant due to the side effects that is life threatening. These countries include Denmark, Norway, Iceland and Thailand for starters.
India is one of the few nations that has undertaken a massive inoculation drive at its hand. It is also out of a very few nations that has been able to develop its own indigenous version of the vaccine which is also being utilized by many of its neighboring nations.
According to an EU regulator, certain kind of allergic reactions are also being observed post the administering of the Astra Zeneca medication. As per the European Medicines Agency (EMA) “an update is recommended to the product information that should include anaphylaxis and hypersensitivity (allergic reactions) as side effects”.
Many Covid-19 variants have also been prescribed as sensitive for those who might be suffering from breathing difficulties or heart issues. Denmark at the moment has suspended the use of the vaccine as cases of blood clot have come to light.
Related Posts
The AstraZeneca vaccine’s product information already said that people should be kept under “close observation for at least 15 minutes” after getting the jab in case of allergic reactions. India seems to be taking a precautionary measure as some nations have suspended the use, despite a WHO advisory that this was unnecessary.
India has already given at least 28 million shots in its vast vaccination programme, most of them being the AstraZeneca variant, which was produced at the Serum Institute of India. As of now, New Delhi has already gifted and allowed exports of millions of these jabs to around 70 countries over the last few weeks as a part of its vaccine diplomacy.



